Figure 1a shows that phosphor ylated FAK localized mainly to the periphery in quiescent NMuMG cells, creating a staining pattern extremely equivalent to that of F actin, which was visualized with phalloidin staining. Even so, upon TGF 1 stimulation, phosphorylated FAK underwent a dramatic reorganization and localized mostly in the end of actin tension fibers. Accordingly, immunoblotting NMuMG cell extracts with a panel of phospho precise FAK antibodies showed that TGF stimulation dra matically elevated the phosphorylation of FAK. Figure 1b also shows that TGF stimulation improved FAK protein selleckchem Panobinostat levels in NMuMG cells and induced an impressive upregulation of three integrin.
Both the boost in FAK phosphorylation and expression, at the same time as the boost in three integrin expression had been wholly dependent on Src activity, simply because treating these very same cells with the Src inhibitor, PP2, abrogated FAK phosphorylation at the Src dependent internet sites and selleck inhibitor prevented TGF 1 induced expression of FAK and 3 integrin. In addition, in contrast to manage and WT 3 integrin expressing NMuMG cells, these engineered to express a signaling deficient mutant of 3 integrin, D119A3 , exhibited drastically lowered mainte nance of FAK protein levels and phosphorylation in response to nonadherent circumstances. To extra completely investigate the role of three integrin in TGF mediated stabiliza tion and phosphorylation of FAK, nonadherent NMuMG cells were replated in the absence or presence of TGF 1 ahead of analyzing FAK expression and phosphorylation by immunob lotting.
As shown in Figure 1d, treatment of manage and WT three integrin expressing NMuMG cells with TGF 1 stimulated increased FAK phosphorylation. In stark contrast, TGF remedy of D119A 3 integrin expressing NMuMG cells in fact decreased their expression and phos phorylation of FAK. Lastly, we conducted real time PCR for FAK in handle, WT3 , D119A 3 integrin expressing NMuMG cells. 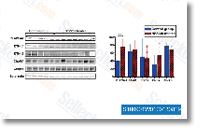 As shown in Figure 1e, chronic TGF stimulation had no effect on FAK mRNA lev els in manage or WT 3 integrin expressing NMuMG cells. on the other hand, these similar experimental situations did raise FAK mRNA expression in D119A 3 NMuMG cells. These data strongly recommend that upregulated three integrin expres sion is needed to stabilize FAK protein levels upon TGF stimulation, and activated three integrin signaling acts as a damaging feedback mechanism governing FAK transcription. Along these lines, our use of oligonucleotide sequences that especially amplified murine three integrin sequences, not that of recombinant human WT or D119A 3 integrin sequences, showed that NMuMG cells engineered to overexpress WT three integrin failed to upregulate endogenous murine 3 integrin transcripts in response to TGF stimulation.
As shown in Figure 1e, chronic TGF stimulation had no effect on FAK mRNA lev els in manage or WT 3 integrin expressing NMuMG cells. on the other hand, these similar experimental situations did raise FAK mRNA expression in D119A 3 NMuMG cells. These data strongly recommend that upregulated three integrin expres sion is needed to stabilize FAK protein levels upon TGF stimulation, and activated three integrin signaling acts as a damaging feedback mechanism governing FAK transcription. Along these lines, our use of oligonucleotide sequences that especially amplified murine three integrin sequences, not that of recombinant human WT or D119A 3 integrin sequences, showed that NMuMG cells engineered to overexpress WT three integrin failed to upregulate endogenous murine 3 integrin transcripts in response to TGF stimulation.
Transcriptase Signal
Telomerases are part of a distinct subgroup of RNA-dependent polymerases.
